Gene therapy has emerged as a groundbreaking approach for addressing a wide array of genetic disorders. With over 30 gene therapies approved by the FDA and more than 2000 in various stages of development globally, the potential for transformative treatments continues to expand. Recombinant Adeno-Associated Viral (AAV) vectors serve as a vital gene delivery mechanism for introducing therapeutic genes into patients. Precise and reproducible characterization of these vectors is crucial to ensure accurate clinical dosing, safety, and therapeutic efficacy. The integrity of the viral genome plays a pivotal role, as variations in genome regions targeted for analysis can influence vector quantitation results, and truncated vectors may fail to produce a functional therapeutic product.
Agathos Biologics recently participated in a panel roundtable discussion hosted by QIAGEN, titled “Viral Vector Integrity Analysis – Advancements in Gene Therapy Development.” The 48-minute session brought together leading experts to explore cutting-edge methods for assessing vector integrity, with a focus on the advantages of digital PCR (dPCR)-based solutions. Key topics included the design of multiplex assays and the challenges involved in their implementation, the significance of reference standard materials and assay qualification, and innovative approaches to genome integrity evaluation. The event concluded with a live Q&A session, providing attendees the opportunity to engage directly with the panel of experts.
Company to show production of multiple serotypes of recombinant adeno-associated viral (rAAV) vectors and rAAV production in suspension-adapted cell line AE1-BHK-S

Agathos Biologics, a genetic medicine biotechnology company, will present data at the 27th Annual Meeting of the American Society of Gene and Cell Therapy on its proprietary AE1-BHK cell line for rAAV production. The poster titled “AE1-BHK Novel Cell Line for rAAV Production of Multiple Serotypes for Gene Therapy Applications”, number 1539, will show data from the adherent AE1-BHK cell line and the poster titled “Establishment of Serum Free Suspension Adapted AE1-BHK-S Novel Cell Line for the Manufacturing of rAAV”, number 1540, will show data from the adaptation of the AE1-BHK cell line to suspension culture, referred to as AE1-BHK-S. These posters will be presented during the poster session “AAV Vectors – Product Development and Manufacturing and Approval Considerations” from 12:00 – 7:00 PM on Friday, May 10.
“We are excited to present data on our continued development of the AE1-BHK cell line,” said James Brown, CEO of Agathos Biologics. “These data show the potential of AE1-BHK as a research tool and biomanufacturing platform. We aim to provide researchers, manufacturers, healthcare providers, and patients, with alternatives for genetic medicine that enhance current approaches and avoid ethical issues. Earlier this year, we launched our custom rAAV production service and fulfilled our first order. We plan to expand our rAAV offerings to include off-the-shelf control and reporter vectors and rAAV reference standards.”
Agathos has submitted a patent application for the cell line and provisional patent applications for related technologies. “We are looking for partners and collaborators who will work with us to develop these products,” Brown added. “Working with those who share our vision of expanding access to these advanced treatments is the best way to make a positive impact on genetic medicine. We will be at booth 2384 at the ASGCT Annual Meeting May 7-11, 2024, in Baltimore, MD, and look forward to discussing our results with attendees.” For more information on the AE1-BHK cell line and to inquire about partnerships, please visit AE1-BHK cell line : Agathos Biologics.
Agathos records first sale of recombinant adeno-associated virus produced using AE1-BHK to collaborator Genovac; provides free access to AE1-BHK cell line
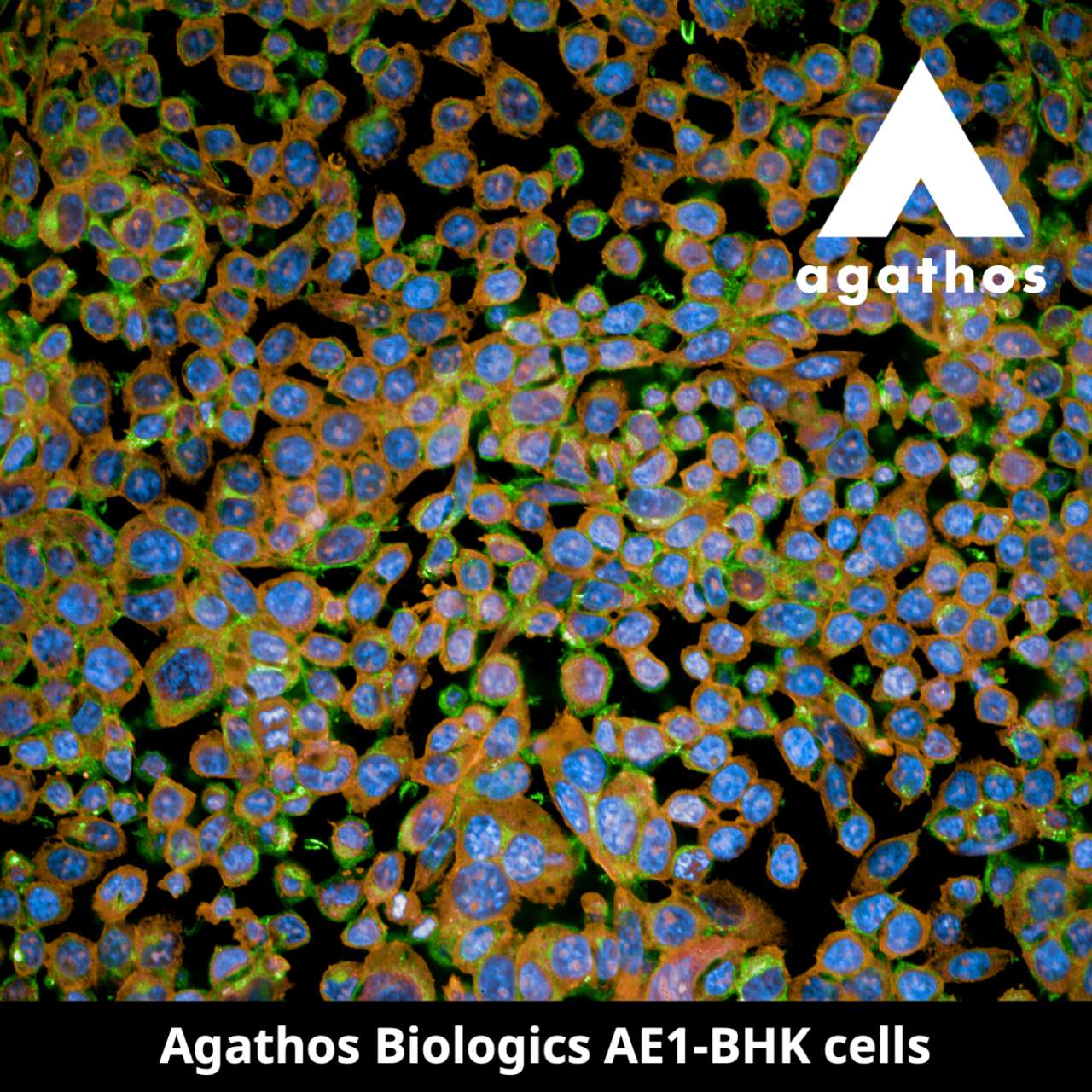
March 6, 2024 (Fargo, N.D.) – Agathos Biologics, a leading biotechnology company in genetic medicine, announced today the launch of its recombinant adeno-associated virus (rAAV) production service using the company’s novel proprietary cell line, AE1-BHK. The company recorded its first sale of rAAV to Genovac, a contract research and manufacturing organization (CRO/CMO) that discovers, develops, and manufactures antibodies for therapeutic, diagnostic, and research market segments. Agathos produced rAAV with a transgene sequence provided by Genovac in AE1-BHK using transfection with three plasmids (triple transfection), a well-established process that has been used extensively with the HEK293 cell line.
The AE1-BHK cell line was developed by stable transfection of the BHK-21 (C-13) cell line (ATCC®, catalog number ATCC CCL-10™) with the adenoviral E1 gene. The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
“The launch of our custom rAAV service and the first sale of rAAV made using our AE1-BHK cell line is a significant milestone for the company,” said James Brown, CEO and co-founder of Agathos Biologics. “To our knowledge, this is the first sale of rAAV produced using triple transfection in a cell line other than HEK293. For our custom services, we will assist clients in the design of a rAAV expression cassette that contains their gene of interest and produce the rAAV for research use both in vitro and in vivo.”
“In addition to our custom rAAV service, we are providing samples of AE1-BHK, a polyclonal line, free of charge,” added Brown. “Clients need only pay shipping and handling to receive these cells for internal research use at their institution or company. Our goal is to make our cells widely available and expand the options for genetic medicine research and development, especially for researchers looking for alternatives to HEK293.”
“Genovac is committed to continuously exploring the most efficient genetic payload systems to illicit immune responses from all animal species and strains,” said Brian Walters, CEO of Genovac. “We look forward to using Agathos’ rAAV as part of our efforts to generate diverse antibody candidates in support of our clients’ research and development needs,” added Walters. “We will also be receiving samples of AE1-BHK for evaluation of its use in recombinant antibody and protein production.”
Agathos is working with multiple collaborators on various aspects of AE1-BHK development. “We continue to develop AE1-BHK as part of our efforts to improve biomanufacturing and avoid ethical issues regarding cell lines derived from aborted fetal tissue like HEK293,” added Brown. “Gene therapy is having a positive impact on addressing unmet medical needs and has only begun to realize its potential. We will engage with others in the industry who share our mission to expand access to these novel treatments through improved manufacturing materials and methods.” Agathos first presented data on AE1-BHK in a poster session at the ASGCT 2023 Annual Meeting (designated BHK-[wt E1] in the poster). The company has submitted patent applications for AE1-BHK and associated technologies. For more information on custom AAV and to request samples of AE1-BHK cells please visit agathos.bio/custom-aav/.
About Agathos
Agathos Biologics is a biotechnology company pursuing transformational science in biomanufacturing, biologic payload delivery, and cell and gene therapy. Discoveries in bioprocessing and genetic characterization and control have created an abundance of scientific possibilities that can help us all lead better lives. Our mission as the good science company is to create breakthrough products and services within a strong ethical and moral framework that benefit everyone. We believe in science that serves and have a relentless focus on serving our clients, employees, and society. For more information, please visit www.agathos.bio.
About Genovac
Genovac is a contract research and manufacturing organization offering advanced antibody discovery and production solutions. Its immunization technologies, combined with multiple single B cell screening technologies, including Bruker’s Beacon, enable success against the most challenging targets. Since its founding in 1999, Genovac has completed more than 3,700 projects, providing antibodies to clients in North America, Europe, Australia, and Asia that have been developed into clinical and commercial drugs. In addition to its headquarters and labs in Fargo, North Dakota, Genovac operates a scientific and production facility in Freiburg, Germany.
September 27, 2023 (FARGO, N.D.) – Agathos Biologics, a biotechnology company developing transformational science within a strong ethical and moral framework, today announced the company has been awarded $300,000 from the 2023 North Dakota Department of Agriculture Bioscience Innovation Grant (BIG) Program. Agriculture Commissioner Doug Goehring announced that nine grants have been awarded totaling $8.4 million to foster the growth of the bioscience industry in North Dakota. “Advances in bioscience have already transformed many sectors including agriculture and medicine,” Goehring said. “These grants will help North Dakota stay on the forefront of bioscience innovation.”
This is the second North Dakota Bioscience Innovation Grant Agathos Biologics has received; the company was the recipient of $900,000 in 2021. The 2021 award provided critical support that allowed the company to launch its analytical services and develop the BHK-[wt E1] cell line for biomanufacturing, furthering the mission of the company to develop tools and technologies for drug development that avoid ethical concerns. This new award, which will fund capital equipment, enables further development of the BHK-[wt E1] cell line and manufacturing materials for sale, as well as our expanding analytical services.
“We are honored to receive this second grant from the State of North Dakota and thank the Commissioner and the Committee for their work on behalf of the citizens of the state,” said James Brown, Chief Executive Officer of Agathos Biologics. “We look forward to continuing to grow in North Dakota and pursue our goal to develop genetic medicine products and services that positively impact human health and are ethically acceptable to all.”
BHK-21 transformed with adenoviral E1 gene and GH329 cell line shown to produce recombinant adeno-associated virus (rAAV)
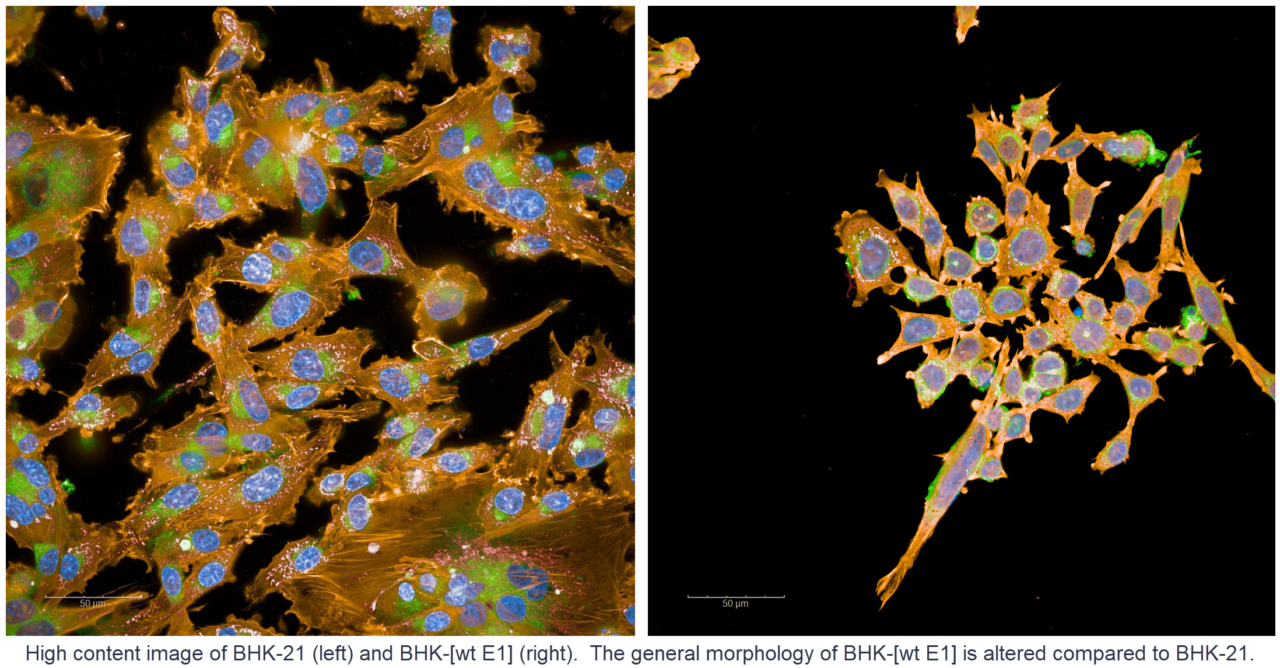
Agathos Biologics, a leading biotechnology company in genetic medicine, will present data at the 26th Annual Meeting of the American Society of Gene and Cell Therapy (ASGCT) from research to develop methods for production of rAAV. The company has developed a cell line based on BHK-21 that it transformed with a plasmid containing the E1 gene of adenovirus. The data show rAAV production with this cell line, Agathos BHK-[wt E1], using a standard triple transfection method. The company will also present data that show a cell line based on HeLa, GH329, which was previously developed for recombinant adenovirus production through transformation using a modified version of the E1 gene of adenovirus, can produce rAAV using triple transfection. The BHK-[wt E1] data will be presented in a poster titled “E1-transformed BHK Cell Lines for Helper Virus-Free AAV Production Using Triple Transfection”, number 1221, and the GH329 data will be presented in a poster titled “Use of the GH329 Cell Line for Adeno-Associated Virus Production”, number 397.
“We are excited to present our data that show rAAV production in Agathos BHK-[wt E1] and GH329,” said James Brown, CEO and co-founder of Agathos Biologics. “Our goal is to provide scientists, manufacturers, health care providers and patients with options for genetic medicine that improve existing methods and address ethical concerns. We are working to develop these and related technologies into products and services for research and biomanufacturing.”
Agathos has submitted provisional patent applications based on these data and associated technologies. The company plans to use the cell lines and methods to produce materials for its internal programs and for sale, initially for research use with expansion into regulated biomanufacturing. “We are actively seeking partners and collaborators who will co-develop these products with us; we believe this is the best strategy to positively impact genetic medicine,” added Brown. “We will be at booth 708 at the ASGCT Annual Meeting May 16-20, 2023, in Los Angeles, CA, and look forward to discussing our results with attendees.” For more information and to inquire about partnerships please visit Agathos Biomaterials.
Initial offerings focus on high content imaging and digital PCR
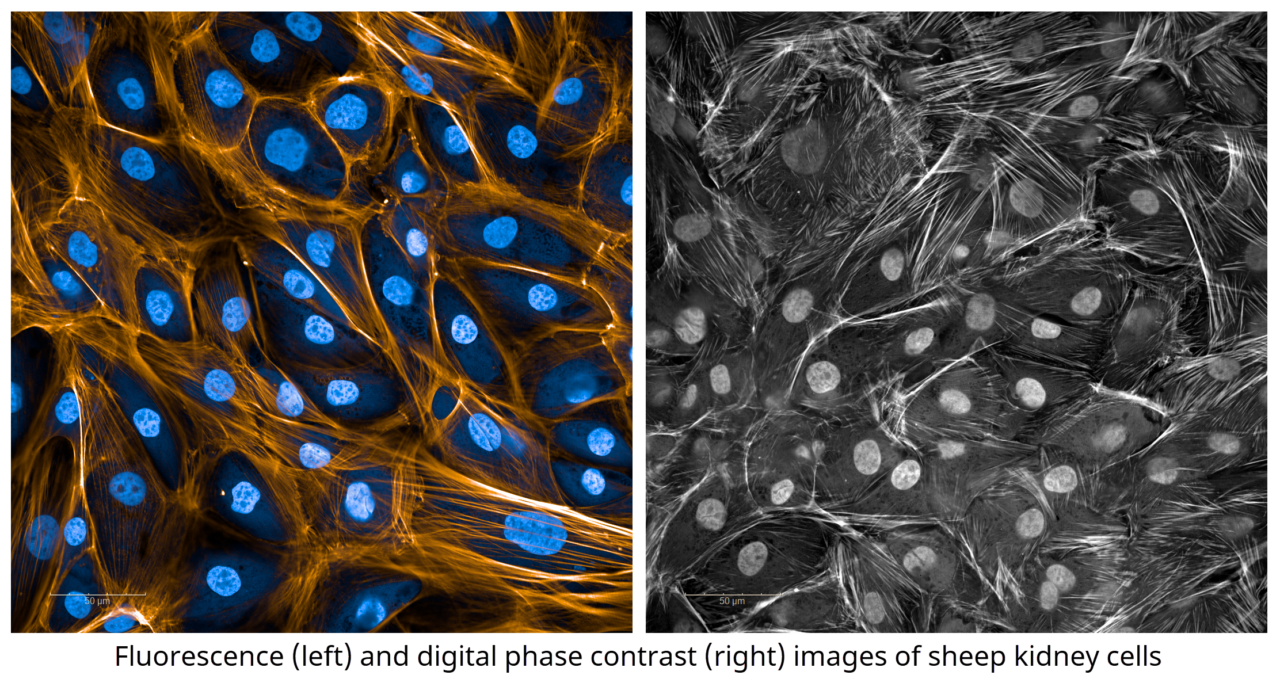
Agathos Biologics, a leading biotechnology company, is proud to offer advanced analytical testing services for life science researchers. With a focus on high content imaging and digital PCR, our team of experienced scientists use state-of-the-art technology to deliver unparalleled accuracy and reliability in nucleic acid quantification and cell analysis.
The first of its kind within the region, Agathos’ high content imaging services allow the capture and analysis of multiple parameters within a cell, providing a more comprehensive understanding of biology than ever before. With various imaging applications, including immunohistochemistry, immunofluorescence, and signaling pathways, our experienced scientists can now offer the best imaging experiments available.
Agathos Biologics uses the latest digital PCR (dPCR) technology, delivering unparalleled sensitivity and precision in nucleic acid quantification. dPCR methods can detect and quantitate low concentrations of target nucleic acid sequences with high accuracy, making it a powerful tool for mutation analysis, viral vector titer determination, and pathogen detection. Agathos exclusively uses QIAcuity instrumentation and assays from QIAGEN and has collaborated with QIAGEN in mycoplasma and recombinant adeno-associated viral vector (rAAV) assay testing and validation.
“Our goal is to advance biotechnology and genetic medicine with products and services through ethical innovation,” said James Brown, CEO and co-founder of Agathos. “As we develop capabilities for our internal research and development, we have the opportunity to make these high-content imaging and digital PCR services available to clients in our region and beyond. These services represent the first step in our strategy to create breakthrough products and services within a strong ethical and moral framework that benefit everyone.” For more information on high-content imaging and dPCR, visit www.agathos.bio/analytical-services.

Agathos was pleased to welcome Mark in January 2022 as the fifth member of its growing team. Joining a small company with ambitious goals required him to come up to speed quickly. “I am excited to be a part of Agathos and create ethically-developed products and services for genetic medicine and biotechnology,” said Voigt. “It has been an intense first few months during which I have immersed myself in the science and strategy of the company. I’m committed to working with the talented founders and team members to create innovative solutions that will positively impact patients, researchers, and healthcare providers.”
Voigt was Director, General and Vascular Surgery at St. Cloud Hospital – CentraCare where he was responsible for physician recruitment and contracting as well as developing new service lines for the health system. Prior to that, he was Key Account Manager at R&D Systems where he worked with scientists, lab managers, and directors to identify products and services to meet their experimental needs for drug discovery, biomarker screening, and translational research.
“Each team member at Agathos must work across functions in a fast-paced environment. Mark has already made significant contributions in a variety of areas, from lab management and procurement to evaluating technology and market opportunities,” said James Brown, CEO of Agathos. “His diverse background in biotechnology sales, product development, and healthcare management brings a set of skills that are important for our success. Mark’s energy and enthusiasm is infectious and has a positive impact on everyone.”
The addition of Mark’s role, the recent addition of laboratory and office space, and the recruiting of new scientist and research associate positions are part of the company’s plan for continued growth in 2022. “The founders of Agathos are dedicated to developing new, ethically-developed tools and therapies for genetic medicine and look forward to growing the company to achieve these goals,” added Brown.

Feb. 23, 2022 (FARGO, N.D.) – Agathos Biologics announced today that it has signed a lease with Comstock Companies to acquire additional laboratory and office space, bringing its total to 10,000 square feet of usable space. “We are excited to expand our footprint in Fargo, North Dakota, which continues to see growth in its biotechnology industry,” said James Brown, CEO of Agathos. “The space is purpose-built for laboratory research, which will allow us to immediately increase our capacity to support our pipeline of projects.”
“We are pleased to see the growth of Agathos, adding to the space leased by life science tenants, including Sanford Health and Immunoprecise, in our research center, the former PRACS building,” said Scott Kjos, Managing Partner of Comstock Companies. “We are eager to support the growing biotechnology industry in Fargo and adding facilities and infrastructure that will enable these companies to have a positive impact on human health.”
Founded by James Brown, John Ballantyne, and Michael Chambers, Agathos Biologics is pursuing opportunities in gene therapy, nucleic acid and protein delivery, and biomanufacturing. Agathos’ mission as The Good Science Company is creation of transformative technologies benefiting patients, physicians, and researchers that are developed ethically, avoiding biological materials from sources including human embryonic stem cells and aborted fetal tissue and procuring materials with proper consent.
“The founders of Agathos believe that the explosive growth of biotechnology enables us to provide ethical alternatives to existing biomaterials that will allow us all to live healthier lives without compromising our deeply held beliefs. We look forward to leading this new approach and working within the industry to realize this vision,” added Brown.
About Agathos
Agathos Biologics is a biotechnology company pursuing transformational science in biomanufacturing, biologic payload delivery, and cell and gene therapy. Discoveries in bioprocessing and genetic characterization and control have created an abundance of scientific possibilities that can help us all lead better lives. Our mission as the good science company is to create breakthrough products and services within a strong ethical and moral framework that benefits everyone. We believe in science that serves and have a relentless focus on serving our clients, employees, and society. For more information, please visit www.agathos.bio.
About Comstock
Comstock Companies provides its customers with leasable space for office, research, commercial, and industrial needs in the tri-state area. Our mission is to serve our customers with the service they deserve.
August 30, 2021 (FARGO, N.D.) – Agathos Biologics, a biotechnology company developing transformational science within a strong ethical and moral framework, today announced the company has been awarded $900,000 from the North Dakota Department of Agriculture Bioscience Innovation Grant (BIG) Program. Agriculture Commissioner Doug Goehring announced that nine grants have been awarded totaling $4.9 million to foster the growth of the bioscience industry in North Dakota. “Advances in bioscience have already transformed many sectors including agriculture and medicine,” Goehring said. “These grants will help North Dakota stay on the forefront of bioscience innovation.”
Agathos Biologics’ project funded by ND BIG will focus on challenges that limit patient access to advanced genetic medicines that can significantly impact quality of life—cost, availability, and ethical concerns. Company scientists will create new materials and methods for research and biomanufacturing and use them for drug development, which will address unmet medical needs and increase the availability of genetic medicines to more patients. The company will make these products and services available to the broader biotechnology industry through direct sales and licensing, partnerships, and collaborations.
“We are honored to receive this support from the State of North Dakota and thank the Commissioner and the Committee for their work on behalf of the citizens of the state,” said James Brown, Chief Executive Officer of Agathos Biologics. “We founded the company in North Dakota because its business-friendly environment, skilled workforce, and growing biotechnology ecosystem make it an ideal place to expand the company and achieve our goal to develop genetic medicine products and services that positively impact human health and are ethically acceptable to all.”
August 26, 2021 (FARGO, N.D.) – As we seek to build Agathos Biologics, it’s important to state our mission, values and strategy. We have taken the first step in this direction, describing our approach on our web site in the pages “Our Mission”, “Our Strategy” and “Good Science for a Better World” (collectively GSBW). We will look for opportunities to refine and further communicate in these areas, and one way to do this is to respond to relevant current events. Recently, Jason Fried, CEO of the software company Basecamp made a public statement about “Changes at Basecamp” and CTO David Hansson followed up with a post titled “new etiquette regarding societal politics at work”, which have garnered considerable news coverage and responses from many different perspectives. We believe Basecamp’s statement raises some important issues that we would like to address in the context of the culture of Agathos Biologics. Read the white paper in full here.